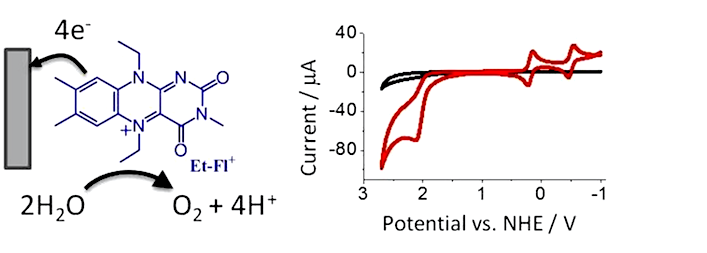
Electrocatalytic Water Oxidation by Iminium Ions
We recently discovered that a fully organic, flavin-based compound (Et-Fl+) catalyzes electrochemical oxidation of water to oxygen. Using electrochemical techniques (cyclic voltammetry, RRDE experiment, bulk electrolysis), we found that catalytic water oxidation occurs at a large overpotential (+1.9 V vs. NHE), with a turnover number of ~13. The catalysis is assisted by certain electrode surfaces and the possible mechanism includes a formation of peroxide between flavin-oxo intermediate and surface oxides. We are currently investigating the mechanism of catalysis by flavinium ion and we are analyzing other iminium ions for potential catalytic behavior. Furthermore, we are interested in developing a fully molecular catalyst that operates without the assistance of the electrode.
"Catalysis of Thermodynamically Uphill Reactions",
ACS Meeting, San Francisco, Fall 2014.
Relevant Publications:
Funding